10 Things About Sushi
At our 2014 Science of Sushi event, Dr. Ole Mouritsen and Chef Morihiro Onodera illuminated the science underlying some of our favorite components of sushi. In case you still haven’t had your fill, here are 10 scientific facts related to sushi:





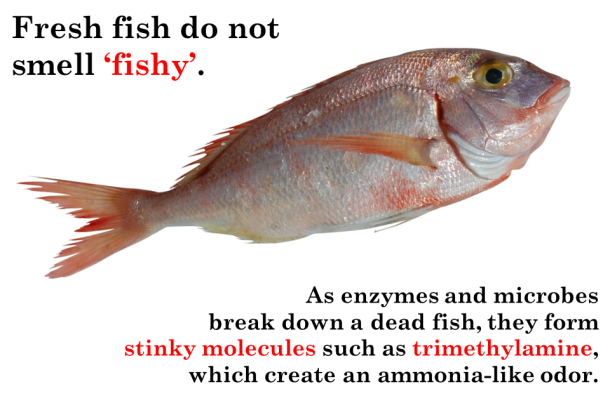
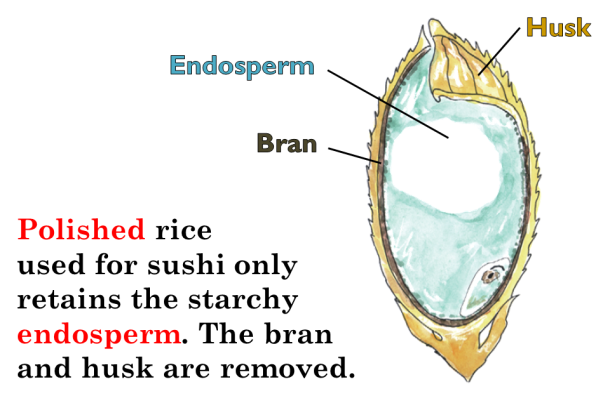



 About the author: Liz Roth-Johnson received her Ph.D. in Molecular Biology at UCLA. If she’s not in the lab, you can usually find her experimenting in the kitchen.
About the author: Liz Roth-Johnson received her Ph.D. in Molecular Biology at UCLA. If she’s not in the lab, you can usually find her experimenting in the kitchen.








