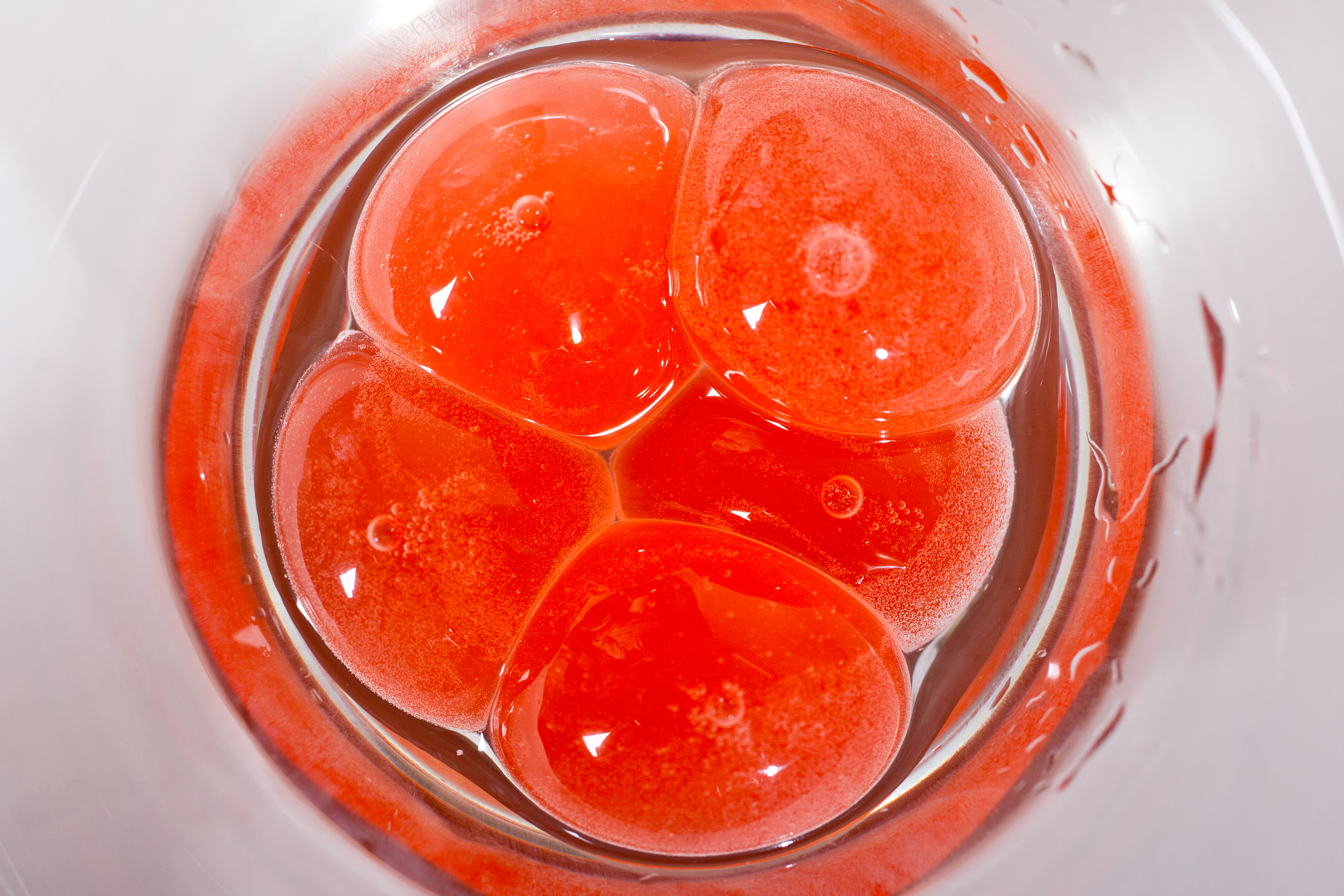
There are times when gourmet edges more towards the laboratory than the kitchen; spherification is one of those times. In this culinary technique, liquids are transformed into globular semisolid gels thanks to a hydrocolloid gum extracted from seaweed. When these gel-encased balls are broken, the liquid contents gush out, akin to biting down on mochi or a Gushers candy. In theory, almost any liquid can be spherified, so the possibilities are endless. Ever wanted to eat plum juice caviar, spherical crème brûlée, or mojito spheres? With food-grade sodium alginate, calcium solution, and some creativity, it’s possible.
At the Spherification Potluck last month, graduate students Liz Roth-Johnson and Kendra Nyberg delved into the process on the molecular level. Gelation is made possible through the interaction between alginate and calcium ions. Alginate is a long, negatively charged, noodle-like molecule. When mixed into a liquid, alginate floats about freely, its elongated structure creating a thick, jelly-like consistency. Calcium ions are single calcium atoms with two positive charges, enabling each ion to link together two alginate molecules. Many calcium-linked alginate molecules gives rise to a more solid structure—the gel skin that encases a gooey center.

Liz (left) and Kendra (right) explain the nuts and bolts of spherification.

Students brought a variety of beverages, sauces, and condiments to the potluck.
Attendees at the student event opted for items found in kitchen pantries and grocery store shelves, such as pomegranate molasses, rose water, coffee drinks, milk tea, sodas, guava nectar, and hot sauce.
In the first attempt at spherification, coffee was mixed with the sodium alginate to produce a rather thick goop. Plopping globs of this dense solution into the calcium chloride baths gave comical results, as the mixture adamantly refused to form any shape remotely resembling a sphere. Some blobs even broke upon removal from the calcium chloride baths.

Students prepare an alginate solution (left) and attempt to create spherified coffee (right)
Milk tea and Jarritos orange soda gave the best results in terms of shape and stability. Initially, the center of the milk tea spheres was thicker than expected, yielding a much chewier texture than bargained for. Minimizing incubation time in the calcium chloride solution managed to fix this halfway, somewhat decreasing the thickness of the gel casing. A quick search also revealed that our recipe used twice the sodium alginate other spherification recipes called for. If less alginate was added to the milk tea or orange soda, the spheres would have definitely been gooier.

A student shows off a fairly successful attempt at spherified orange soda.
The most difficult to work with was Tapatio, and not just because of the spicy fumes that emanated from the mixing bowl. Hot sauce is acidic, meaning it is full of positively charged hydrogen ions. Mixing it with alginate neutralizes the negative charges, hampering the interaction between alginate and calcium. No alginate-calcium interaction, no cross-link formation, no gel. Dropping the Tapatio-alginate mixture into calcium chloride resulted in nothing more than dissolved Tapatio swirling around in solution.
Spherification encompasses a high degree of flexibility. Besides the gamut of foods that can be used, there are also technical alterations—the ratio of liquid to sodium alginate in the pre-sphere goop; the concentration of the calcium chloride solution; the amount of time the spheres are left sitting in the calcium solution. And this is only the direct method. Other variations on this technique include reverse and frozen reverse spherification. With spherification kits readily available online, why not try spherifying your own recipe? Share your spherification adventures with us in the comments below!