Ginger
One rhizome, many tastes. Ginger can be charmingly sweet as candied ginger, gingerbread, and ginger ale. Just as easily, this root can be spiritedly pungent, as in gari (sushi ginger) or unsweetened ginger tea. From sugary snacks to savory dishes, ginger shares similar flavor versatility as cardamom, which should come as no surprise; the two spices are practically cousins. All ginger plants are of the genus Zingiber, which belongs to the same family as cardamom plants, Zingiberaceae [1]. However, the supermarket ginger that most people are familiar with is the knobby, root-like rhizome of Z. officinale, better known as the garden ginger.
Fresh ginger gets its pungency and aroma from the flavor compound, gingerol. Studies have extolled gingerol for its many pharmacological abilities, including antipyretic (fever reducer), analgesic (pain reliever), anti-inflammatory, and antibacterial [2]. The best part? Chemically altering gingerol ends up tweaking ginger’s flavor profile, which helps give ginger its flavor versatility. No laboratories or fancy equipment are needed; as long as there’s a kitchen and a love for ginger-flavored foods, fine-tuning the flavor of ginger is rather straightforward.
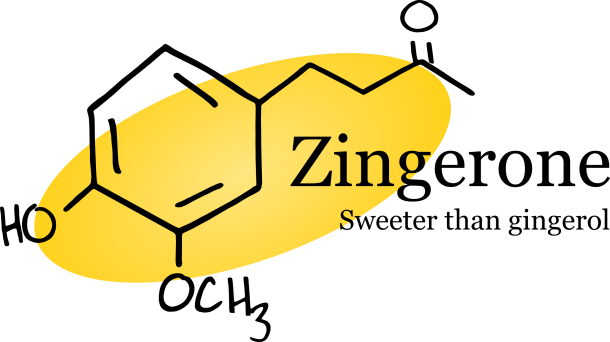
Heating a ginger rhizome causes gingerol to undergo a reverse aldol reaction, transforming it to zingerone, a molecule that is completely absent in fresh ginger. Like gingerol, zingerone is responsible for the pungency of cooked ginger, but it also lends a sweeter note to the flavor. For this reason, cooked ginger makes a delightful treat as candied ginger. Zingerone also boasts quite a few pharmacological benefits, notably, its many anti-obesity actions [3]. For instance, zingerone was shown to inhibit obesity-induced inflammation, as well as stimulate the release of catecholamine, a hormone that aids in decreasing fat cells [3].
Drying a piece of ginger triggers a dehydration reaction, changing gingerol to shogaol. Shogaol is twice as spicy as gingerol, which is why dried ginger packs more heat than its fresh counterpart. Additionally, shogaol retains gingerol’s bioactivity, reported to act as an antioxidant, anti-neuroinflammatory, and even memory-enhancing agent [4].
With a multitude of benefits and just as many ways to serve it, there’s really no wrong way to enjoy ginger.
References cited
- Zingiber. The Plant List (2010). Version 1. Published on the Internet; (accessed 13 August, 2014).
- Young H.-Y, et al. Analgesic and anti-inflammatory activities of [6]-gingerol. Journal of Ethnopharmacology. Jan 2005; 96(2):207-210.
- Pulbutr P. et al. Lipolytic Effects of zingerone in adipocytes isolated from normal diet-fed rats and high fat diet-fed rats. International Journal of Pharmacology. Jul 2011; 7(5):29-34.
- Moon M, et al. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochemical and Biophysical Research Communications. June 2014; 449(1):8-13.
 About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.
About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.