Tag Archive for: milk
Anatomy of a hot chocolate
/in Science & Food/by Grant Alkin
Photo credit: Flickr/louish
Hot chocolate: it’s a winter staple. Amidst falling temperatures and dreary skies, there’s nothing quite like taking a swig of this sumptuous beverage and seeking warm refuge in the delights of a steaming mug. Hot chocolate is as straightforward as drinks go: at its core, it’s milk, cocoa powder, and sugar. Despite its simplicity, this cold-weather classic is swirling with science.
The backbone of any decent hot chocolate is milk. Beyond water, milk is perhaps the most basic and familiar substance to humans. We’re all born drinking some form of it, but how often do we stop and think about its underlying science? Milk is an emulsion, which is a mixture of two immiscible liquids—in this case, water and fat. The water-based component of milk is loaded with vitamins, minerals, and protein and contains immiscible fat globules suspended throughout. How do water and fat coexist peacefully in solution together? The answer lies in emulsifiers, which are molecules that are both water- and fat-soluble. Milk contains proteins, namely casein, that attract and unite the fluids that would otherwise separate. Rich, silky, and chemically intriguing, this dairy product serves as the perfect vehicle for chocolate (1).

Photo credit: Flickr/chocolatereviews
Chocolate serves as the heart of the beverage. Some recipes call for it in the form of cocoa powder. Cocoa powder mixed in with your milk is a colloid—a type of mixture in which solid particles are dispersed throughout a fluid. Another popular culinary colloid you may recognize is coffee, which contains small coffee particles dispersed in water.

Photo credit: Flickr/csb13
A glass of hot chocolate simply isn’t complete with a dollop of whipped cream plopped on top. Lauded for its decadent mouthfeel, cream is an emulsion of butterfat and water, similar to milk but with a higher fat content. Fresh milk left undisturbed will separate into two layers; the top becomes enriched with fat globules that can be skimmed off as cream, leaving behind a relatively fat-free layer—skim milk. Cream and milk have remarkably different fat contents, as cream is required to have at least 30% milk fat compared to whole milk which is a mere 3%.
With some simple agitation, willpower, and a whisk, we can transform heavy cream into whipped cream, a culinary foam. Similar to emulsions, foams combine two immiscible substances, but instead of water and fat, air or gas is entrapped within a fluid or solid. Whisking incorporates air into the cream, and the newly introduced bubbles are held captive by the structure of the foam. Fluids and gases have very different properties, so how does agitation keep them together? Agitation disorients the fat globules and strips away their protective membranes, forcing them to cling to other fat molecules or aggregate around air bubbles—anything to avoid having to be in contact with water. Agitate your cream enough and you’ll wind up with stiff peaks when these fat-encapsulated air bubbles begin to form a stable network (2).

Photo credit: Flickr/knitsteel
Whether they’re being roasted over a campfire or floating lazily on the surface of your hot chocolate, marshmallows are a surefire way to please and are another way to enhance your chocolate-drinking experience. Marshmallows were originally made as a meringue (yet another culinary foam!) consisting of whipped eggs and sugar flavored with the juice from roots of the marsh mallow plant. The making of marshmallows has since evolved so that now they are created by aerating a mixture of simple sugar syrup and gelatin to form a foam that stabilizes once the gelatin sets. Whipping incorporates air bubbles that are trapped in the solid matrix, forming these springy and sugary confections that pair exceptionally well with chocolate (1).
Hot chocolate is the ultimate winter beverage. It’s creamy, decadent and versatile. Drink it plain or spice it up with some chili powder, orange, or peppermint and you’ll surely find a style that will leave you positively foaming at the mouth.
References cited
- McGee, Harold. On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004. Print.
- Lower, Claire. Cream Science: On Whipping, Butter, and Beyond. Serious Eats. 2014.
 About the author: Mai Nguyen is an aspiring food scientist who received her B.S. in biochemistry from the University of Virginia.
About the author: Mai Nguyen is an aspiring food scientist who received her B.S. in biochemistry from the University of Virginia.
The Keys to Cheese: Does This Cheese Melt?
/in News & Views/by Grant AlkinMelted Cheese Frize [Photo Credit: Pittaya Sroilong]
Whether you are making cheese fries, grilled cheese sandwiches, quesadillas, baked cheese bites, or homemade mac and cheese, choosing the right type of cheese can make or break these comfort foods. The key to all of these dishes is cheese that produces an even and homogenous melt. Cheeses like Cheddar, Mozzarella, and Gruyere are used often. If you aren’t feeling adventurous, you could just memorize the names of these greatest hits. However, if you want to experiment and change the melty cheese game, you’re going to have to understand why these cheeses work.
Let’s first examine what happens to cheese as it melts. The interactions of casein (milk proteins) and calcium help define its solid structure. When solid, caseins are bound together in large branching porous protein networks that entrap milkfat and water. Calcium (as calcium phosphate) acts as a bridge to stabilize these networks. When you apply heat to a cheese, melting occurs in two stages. First, at around 90 ˚F, milkfat is released1. This is because hydrophobic (water-repulsive) interactions between casein molecules increase under heat2. These interactions force out water molecules and the space between casein molecules increases allowing milkfat, which melts at this temperature, to escape. If you’ve put cheese on a burger that’s being grilled, you may see little sweat beads of liquid form on the cheese in the early stages of melting. The second stage happens at about 40 to 90 degrees higher, at around 130 – 180˚F3. At this point, the casein proteins do not break down, but rather, the increased movement of the proteins, resulting from the heat, allows for the proteins to act more fluid-like and the cheese melts.
There are many factors that control melting and explain why melting temperatures vary by as much as 50 degrees. No one factor defines a cheese’s melting properties as these factors can interact.
Moisture and Fat
Cheese with higher moisture and fat content tends to have lower melting points. For example, high moisture cheeses like Mozzarella melt around 130 ˚F and low moisture cheeses like Swiss melt at 150 ˚F 2. First, as previously highlighted, the milkfat and water portion of the cheese react to heat at lower temperatures than the proteins. Accordingly, with more moisture and fat present in a cheese, greater proportions of the cheese are susceptible to melting at lower temperatures. When the fat becomes liquid, it can no long provide support for the protein networks. Secondly, increased moisture and fat means that the casein proteins are more spread out and the mesh size (gap between proteins) is larger. This means there are fewer connections (bound calcium bridges) between proteins networks making melting more likely to occur at lower temperatures.
You may not know the exact moisture and fat content of every cheese variety without looking at a label, but intuitively, softer cheeses have more moisture and fat. Additionally, younger cheeses generally have more moisture so they also tend to melt more uniformly and evenly.
Acid Content
Chesses typically melt homogenously and evenly around a pH of 5.0 – 5.44. This is related to the calcium bridges. At too high a pH (pH > 6), too much calcium is present as bound calcium phosphate and the protein is too tightly bound to melt. With lowered pH, the calcium phosphate bound to the casein is replaced by hydrogen (H+), allowing for more movement among proteins.2 At around a pH of 5.0 – 5.4, there is a sufficient number of calcium present as bridges to allow for melting. At too low a pH (pH < 4.6), too many calcium bridges are lost and proteins aggregate and are unable to flow and melt evenly.
Lastly as a caveat, the factors being highlighted are specific to rennet-set cheeses, and not acid-set cheeses. Acid-set cheeses like queso fresco, paneer, and ricotta are not generally used, as they don’t produce even melts4. This results from the way they were made. In cheese making, you have two options for separating the solid curds (primarily casein proteins) and the liquid whey; Use rennet (an enzyme derived from the intestines or baby goats and cows) or use an acid (like vinegar or lemon juice).
When they are free floating in liquid milk, casein proteins have a slightly different molecular structure than when they are in cheese. In milk, caseins stick together in small clusters (micelles) that have negative charges on their surface. Since negative charges repel each other, these micelles won’t combine. Adding acid to heated milk lowers the pH, which neutralizes the negative charges on the micelles; therefore the casein micelles can aggregate. In contrast, using rennet to set cheese is a more targeted approach. In this process, an enzyme contained in rennet called chymosin, selectively removes negatively charged portions of the casein micelles and allows the micelles to clump.
In an acid-set cheese, calcium bridges are never formed as a result of the acidic environment used to generate the cheese5. These cheeses are only held together in protein aggregates rather than protein networks with calcium bridges and don’t produce the even melt desired.
Bottom Line:
Rennet-set cheeses with high moisture and fat are the best cheeses for melting as they melt evenly and consistently.
But don’t fret if you still want to harness the flavor of other cheeses (especially older or drier cheeses)! You have options: Try using a cheese blend with a higher proportion of the better melting cheeses and a small proportion of the other cheeses. For example, this recipe uses a 1:4 ratio. Experiment! You now know the keys for melty cheese!
References cited
- Schloss, Andrew and David Joachim. “The Science of Melting Cheese” http://www.finecooking.com/item/64019/the-science-of-melting-cheese
- Johnson, Mark. “The Melt and Stretch of Cheese” https://www.cdr.wisc.edu/sites/default/files/pipelines/2000/pipeline_2000_vol12_01.pdf
- Mcgee, Harold. On Food and Cooking. 2004 “Cheese” (57 – 67).
- Tunick, Michael. The Science of Cheese. 2013 “Stretched Curd Cheeses, Alcohols, and Melting” (82 – 91).
- Sargento Food Service. “Cheese Melt Meter” http://www.sargentofoodservice.com/trends-innovation/cheese-melt-meter/
- Achitoff-Grey, Niki. “The Science of Melting Cheese” http://www.seriouseats.com/2015/08/the-science-of-melting-cheese.html
 About the author: Vince Reyes earned his Ph.D. in Environmental Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.
About the author: Vince Reyes earned his Ph.D. in Environmental Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.
Structural Changes in Chocolate Blooming
/in News & Views/by Grant AlkinIs there anything more disappointing than finding a chocolate bar in the back of the desk drawer, anticipating a tasty treat, then unwrapping the bar only to find a dull, grey haze has overtaken your dear candy? Seeing as bloomed chocolate is still edible, yes, there are many things more disappointing than that. But surely you’re curious about how chocolate that was once shiny and perfect came to be filmy and rough. Chocolate blooming, the process that produces the white-grey film that appears on the surface of an old chocolate, is due to molecular migration. More specifically, this imperfection is caused by the movement of fats to the surface of the chocolate followed by a subsequent recrystallization. In a paper published by Applied Materials & Interfaces, a team of researchers dedicated to keeping our chocolates blemish-free has clarified the precise mechanisms that cause chocolate blooming.
The main fat in chocolate is cocoa butter, which is solid at room temperature and melts at 37 degrees Celsius. The proportion of solid to liquid cocoa butter depends on the lipid composition, which depends on which specific triglycerides are present. The solid to liquid proportion also varies with the storage conditions of the chocolate.
As proposed by Aguilera et al, scientists who study this chocolate blooming, consider chocolate as a particulate medium of fat-coated particles such as cocoa solids, sucrose, and milk powder, all suspended in a fat phase with the aid of an emulsifier, which helps to mix fats and oils with water, which usually repel each other. There are six crystallographic polymorphs of cocoa butter molecules, that is, there are six ways the molecules can organize themselves. The structural stability of these polymorphs increases from 1- 6; form 1 is the best at forming solid butter at room temperature, while form 6 tends to arrange in the loose bonds of a liquid. Form 5 is the main form in chocolate, as it possesses the most aesthetically desirable properties. While the phenomenon of blooming is well known to result from melting and recrystallization of chocolate into a less desirable polymorph, it has been unclear how fat moves through the chocolate particle network: Does it move along the fat-particle interface? Does it diffuse through the fat phase (cocoa butter), or through the matrix of assorted particles?
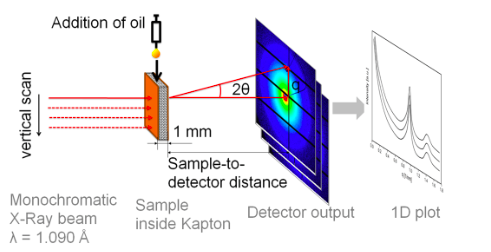
In this experiment, researchers used synchrotron microfocus small-angle X-ray scattering to determine the preferential migration pathway of the cocoa butter molecules surrounded by three different soild components (cocoa solids, skim milk, and sucrose). This technique allows researchers to record the scattering of x-rays through a sample with defects in the nanometer range. They can then extrapolate information about the material’s macromolecules, their shapes and sizes up to 125 nanometers, and distances between partially ordered materials, such as pore sizes. For this experiment, this method is better than more traditional macroscopic techniques as the sample does not need to be dissected in order to examine it, therefore the same sample can be continually analyzed.
The researchers prepared and tempered four different chocolate samples. An initial scattering of x-rays and data collection was performed before the addition of sunflower oil, then 10 uL of oil was pipetted onto the chocolate surface, and a second scan was performed. Images of the droplet were captured through a high-speed camera. These scans were repeated at 5, 10, and 30 minutes after oil addition, and again after 1, 2, 5, and 24 hours.
The results obtained suggest that oil is migrating through pores and cracks in the solid structure driven by capillarity within seconds. This means that the oil can flow in narrow spaces in opposition to gravity. Then chemical migration through the fat phase occurs. The oil doesn’t traverse the fat-particle interface, nor does it move through the matrix of solid particles. This migration disrupts the crystalline cocoa butter, which induces softening.
Because the most immediate migration of oils occurs through the material porous structure, the formation of chocolate bloom could be prevented by minimizing pores and defects in the chocolate matrix. To prevent the longer-term effects of chemical migration of lipids, one must minimize the content of non-crystallized liquid cocoa butter. Tempering chocolate lends to crystalline structures that resist migration, as will reducing the liquid fat content. However, to ensure that you never encounter a sad hazy chocolate again, we recommend eating all chocolate goods expeditiously.
Works Cited
- Tracking Structural Changes in Lipid-based Multicomponent Food Materials due to Oil Migration by Microfocus Small-Angle X-ray Scattering. Svenja K. Reinke, Stephan V. Roth, Gonzalo Santoro, Josélio Vieira, Stefan Heinrich, and Stefan Palzer. ACS Applied Materials & Interfaces 2015 7 (18), 9929-9936. DOI:10.1021/acsami.5b02092
- Aguilera, J. M.; Michel, M.; Mayor, G.Fat Migration in Chocolate: Diffusion or Capillary Flow in a Particulate Solid?—A Hypothesis PaperJ. Food Sci. 2004, 69, 167–174
 About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.
About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.
The Science of Steamed Milk: Understanding Your Latte Art
/in Science & Food/by Grant AlkinGuest post by Christina Jayson
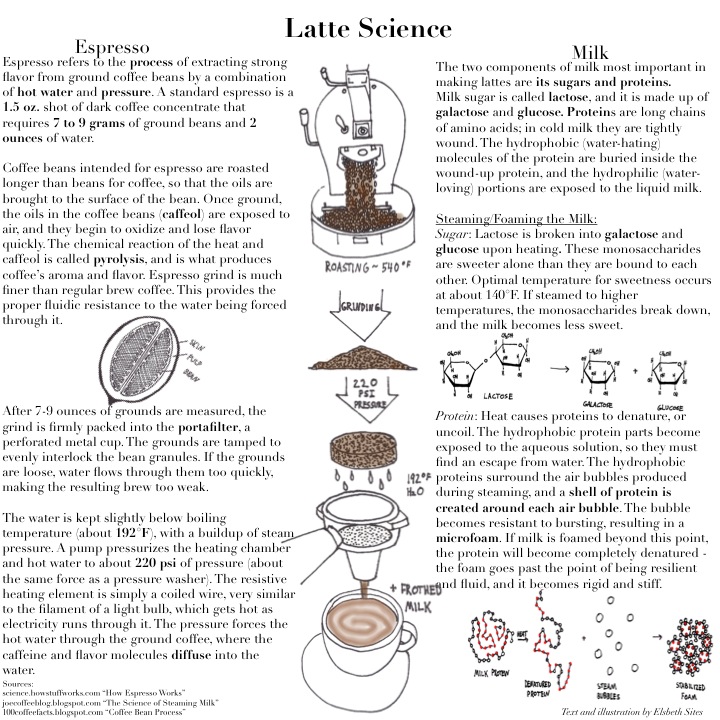
Watch a barista at work and you will observe the art of preparing a perfect café au lait, cappuccino, macchiato, or mocha – all of which involve different quantities of steamed milk. Behind the artistic foam hearts and milk mustaches lies a science to steamed milk.
Students of UCLA’s SPINLab (Simulated Planetary Interiors Lab) team developed an app that allows you to “calculate the power output of your steamer” and predict the “steaming time for optimal milk temperature based on amount, type and starting temperature of your milk”. Samuel May of SPINlab explains the calculations the app takes into account that allows it to predict the temperature of milk at a given time. They show that the temperature increase of milk over time is linear, allowing them to make these predictions based on a Linear Heating Model.
But what exactly happens when you steam milk? Steaming involves introducing hot water vapor (T = 250-255 °F) into cold milk (T = 40 °F) until it reaches the ideal temperature for a “perfectly steamed latte.”
While the process sounds simple enough there are a host of variables that need to be considered. Most importantly, different milks require different amounts of steaming time. As SPINLab expert, Sam warned, too high a temperature can scald the milk: scalding kills bacteria and denatures enzymes; this inactivates the enzymes and causes curdling as denatured milk proteins clump together. Since different types of milk and dairy alternatives have different molecular compositions, this means they have different steaming temperatures. This difference all boils down to the composition of milk.

Figure 1. Milk broken down into its molecular constituents. Modified from Properties of Milk and Its Components. [3]
Milk is 3.3% total protein, including all nine essential amino acids; the protein content can be broken down into two main types, casein and serum. Serum, or whey proteins, contain the majority of the essential amino acids. Whey proteins can be coagulated by heat and denaturation of some of these proteins with heat; this gives cooked milk a distinct flavor. Caseins form spherical micelles that are dispersed in the water phase of milk [1]. When steaming milk, the injected air bubbles disrupt the micelles. The protein molecules then encompass the air bubbles, protecting them from bursting and leading to the formation of foam. The take away: The different protein content of different milks consequently affects each milk’s ability to maintain that frothy foam decorating your latte [2]. Whole milk results in a thicker, creamier foam and skim milk results in more foam and larger air bubbles, while almond milk is able to hold a light and long-lasting foam [2].
Table 1: Percent of protein in different types of milk and non-dairy alternative [2]
| Milk | % Protein |
| Skim milk | 3.4 |
| 1% milk | 3.4 |
| 2% milk | 3.3 |
| Whole milk | 3.2 |
| Soy milk | 2.7 |
| Almond milk | 0.4 |
Lactose is the carbohydrate component of milk – a disaccharide composed of D-glucose and D-galactose. There are two forms of lactose present in an equilibrium mixture due to mutarotation, α-lactose and β-lactose. β-lactose is the more stable form, and also the sweeter form of the two [3]. When you steam milk past a temperature of 100 °C, this causes a “browning reaction,” or the Maillard reaction, in which the lactose and milk proteins – mostly caseins – react to form what is know as an Amadori product [4]. The colorless Amadori product is a molecular complex between the lysine residues of protein molecules and the lactose molecules. As the reaction continues with heating, the Amadori product can undergo dehydration and oxidation reactions, or rearrangements that lead to a loss of nutritional value and the formation of unappealing flavor compounds in milk that Sam warned could result from over-steaming.
The last main constituent of milk is the milkfat that exists as globules in the milk. Over 98% of milkfat is made up of fatty acids of different types, including saturated, monounsaturated, and polyunsaturated fatty acids. These fat molecules can also stabilize the formation of foam by surround the air and entrapping it in a bubble. While higher fat content leads to stable foam at temperatures below room temperature, milks with lower fat contents (like skim milk) are better at stabilizing foam at higher temperatures [3]. This could be due to the reduced surface tension of the fat along the air bubble surface that is a result of an increase in fat percentage. Heating up these fat molecules not only affects foam texture; when heated or steamed, the fatty acids also participate in chemical reactions, such as oxidation reactions, that can give rise to an undesirable flavor [5].
For the lactose intolerant and fans of non-dairy alternatives, you may be wondering how lactose free options such as soy or almond milk compare. Their steaming temperatures differ mildly due to their distinct properties – for example, almond milk has a lower protein content (Figure 2). According to the experience and experimentation of expert baristas, certain brands of soy or almond milk can hold a foam better than others; the science underlying this phenomenon still remains to be determined.
Table 2: Ideal steaming temperatures for milk and non-dairy alternatives [6]
| Milk | Soy Milk | Almond Milk | Coconut |
| 150 °F | 140 °F | 130 °F | 160 °F |
The moral of the story is that each component of milk contributes to its ability to froth and foam, and steaming influences each of these components. With this knowledge, you can wisely choose your milk at Starbucks depending upon your foaming desires, or simply download Sam’s app and perfectly steam your milk at home.
References cited
- O’Mahony, F. Milk constituents. Rural dairy technology: Experiences in Ethiopia, Manual No.4; International Livestock Centre for Africa Dairy Technology Unit, 1988.
- Blais, C. The Facts About Milk Foam. Ricardo, [Online] November 2014;
- Chandan, R. Properties of Milk and Its Components. Dairy-Based Ingredients.; Amer Assn Of Cereal Chemists, 1997; pp 1-10.
- van Boekel, M.A.J.S. Effect of heating on Maillard reactions in milk. Food Chemistry. 1998, 62:4, 403-414.
- Walstra, P. Dairy Technology: Principles of Milk Properties and Processes; CRC Press, 2013.
- Dairy Alternatives – Soy, Almond, Coconut, Hazel, Cashew. Espresso Planet. [Online] April 2013;
Christina Jayson is a recent UCLA Biochemistry graduate about to embark on her Ph.D. journey at Harvard.
International Variations of Yogurt: A Cultural Exploration of Milk
/in Science & Food/by Grant Alkin
Photo credit: Robert Koehler
It’s a dessert, it’s a condiment, it’s a breakfast staple. Yogurt can be consumed in a myriad of ways; there also exist several variations of yogurt around the world that differ dramatically in taste and texture.
It all begins with milk—be it from a cow, sheep, buffalo, donkey, or goat. In standard western forms of yogurt, the art of yogurt-making begins by heating milk to 85°C and holding for 30 minutes or at 90°C for 10 minutes. Applying heat denatures the whey protein, lactoglobulin, and plays a pivotal role in determining the yogurt’s final creamy texture. Without this protein denaturation, the milk proteins won’t set together in an organized matrix, but will instead cluster together and form curds.
In the second phase, fermentation, milk is first cooled to a temperature within the range of 30°C-45°C, a range well tolerated by the microbes that play a role in fermentation. Traditional yogurt-making in the western world relies on bacterial cultures containing Lactobacillus bulgaricus and Streptococcus thermophilus, which can be directly added to milk in the form of a packaged starter (similar to yeast) or taken from a previously-made batch of yogurt. The bacteria then convert the milk sugar, lactose, into lactic acid, which firms the yogurt and provides it with its tart and tangy taste.
Fermentation conditions heavily influence the flavor and consistency of the final product. Different types of bacteria thrive in different temperature ranges and can produce several variations in consistencies, ranging from smooth and creamy to thick and jelly-like. At lower temperatures, bacteria produce lactic acid much more slowly and it can take up to 18 hours for the yogurt to set; by contrast, lactic acid bacteria working at higher temperatures can set the milk proteins in just two or three hours. A rapid, high-temperature gelling will result in a firm yogurt, whereas a low-temperature, slow gelling will produce a more delicate, tightly packed protein network. [1]
Throughout the world, we’ll find several different kinds of yogurt or yogurt-like products. Significant textural or taste changes can be made with simple tweaks in the preparation or fermentation process, and we’ll explore a few of these here.
Greek yogurt
Greek yogurt has become ubiquitous in grocery stores over the past decade and is loved for its rich flavor and thick consistency. The secret behind its popularity lies in the straining step. Straining allows the liquid whey component of milk to drain away and also removes some of the lactose, leaving behind a product with reduced sugar and nearly double the protein when compared to its non-strained counterparts. Although not actually of Greek origin (its origins still remain unclear), this strained yogurt is also popular throughout the Middle East and Central Asia [1,2].
Viili
This slimy dairy product topped with mold may sound like a failed kitchen experiment, but is in fact a yogurt-like product held dear to many Scandinavians. Known as viili to the Finnish, långfil to the Swedes, or tättemjölk to those in Norway, this ropy milk product is so thick that it needs to be cut with a knife when served. The main culprit in viili’s ropiness is a mesophilic strain of lactic acid bacteria called Lactococcus lactis subspecies cremoris. During fermentation, this strain of lactic acid bacteria produces long strands of slimy sugars known as exopolysaccharides that create the characteristic texture and flavor profile of viili. On its surface, you’ll find a velvety layer of mold formed from G. candidum, which lends this dairy product fruity and savory notes. The mold also consumes lactic acid, reducing the acidity of viili, giving it a relatively mild acidic flavor. To make viili, you’ll need milk and a viili starter, which contains both Lactococcus cremorisx and G. candidum [3].
Kefir
Although not technically yogurt, kefir is a bubbly, mildly alcoholic, Russian-derived equivalent. What distinguishes kefir from yogurt is that instead of relying solely on lactic acid bacteria for fermentation, it’s made from kefir grains, which are large cauliflower-like complexes composed of lactobacilli bacteria and yeasts. Kefir also sets itself apart from other fermented milk products in that its fermenting microbes exist in these relatively large, popcorn-sized ‘grains,’ instead of being evenly dispersed throughout the milk. While bacteria are busy converting a portion of the lactose into lactic acid, yeasts from the kefir grains also convert lactose into ethanol and carbon dioxide. The result is a tangy, yeasty, and effervescent beverage [4]. Kefir boasts higher probiotic activity than typical yogurts, making it a fermented dairy product of choice among health enthusiasts.
Ayran
This national drink of Turkey is made by diluting natural yogurt with ice water and salt. Throughout Turkey, different regional variations of ayran exist, with the most well-known version originating from a town called Susurluk. In Susurluk, local variations of ayran are made from a mixture of cow, buffalo, and sheep’s milk, giving it a distinctive creamy and foamy quality. A notable feature of Ayran is its lower shelf-life when compared to other fermented milk products. Stabilizers are added to ayran to prevent the water and milk mixture from separating, but salt hinders the effects of stabilization [5].
Yakult
Now found all over the world and most notably in vending machines throughout Asia, Yakult is a probiotic drink that was developed in Japan. Japanese microbiologist, Minoru Shirota, was searching for a strain of bacteria that would benefit digestive and overall health. His work led him to Lactobacillus casei Shirota, which he cultivated and used to develop Yakult. Yakult is made from adding this unique bacteria strain to skim milk, water, and sugar, and is often enjoyed for its sweet and fruity natural flavors. As seen above, Yakult can also be found in several flavor variations as well.
Cultured milks are a culinary marvel, especially when you consider how many different forms it can take. Head to the dairy aisle and try a new variation of yogurt, or why not attempt making some at home in your own kitchen? With all the different forms out there, you’ll surely find one you enjoy.
References cited:
- McGee, Harold. On Food and Cooking. The Science and Lore of the Kitchen. New York: Scribner, 2004. Print.
- Lalime, Jennifer. “How to Make Greek-Style Yogurt“. The Feed.
- Salminen, Edith. “There Will be Slime“. Nordic Food Lab.
- Farnworth, E. R. Kefir—a complex probiotic. Food Science and Technology Bulletin.
- “Fame of Foamy Ayran Goes Beyond Borders“. Hurriyet Daily News.
 About the author: Mai Nguyen is an aspiring food scientist who received her B.S. in biochemistry from the University of Virginia. She hopes to soon escape the bench in pursuit of a more creative and fulfilling career.
About the author: Mai Nguyen is an aspiring food scientist who received her B.S. in biochemistry from the University of Virginia. She hopes to soon escape the bench in pursuit of a more creative and fulfilling career.
Cheese Holes & “Imaginary Meals”
/in What We're Reading/by Grant AlkinAs it turns out, the holes in Swiss cheese may be from hay particles found in milk, say Swiss scientists. Also, scientists in California have developed a diet drug called Fexaramine, which may trick the digestive system into burning fat without food. Read more
Garlic
/in Flavor of the Month/by Grant AlkinIf you’ve ever made the mistake of devouring three bowls of James Beard’s Garlic Soup a few hours before The Job Interview Of Your Life (I’m not speaking from experience here), you will recognize the frantic moment in which you pray that 1) the handful of mints burning in your mouth have superpower strength, or 2) your interviewers cannot smell, or 3) whoever you’re meeting had four bowls of garlic soup. Ahhh, the allure and woe of garlic. Why do you hate me if I love you so much?
Known for its distinct aroma and taste, Allium sativum – or garlic, as most of us know it – makes dishes sweet and pungent while it turns breaths foul and fetid. But what exactly causes garlic breath? More importantly, how do you get rid of it?
The Breakdown of Garlic Breath
Garlic contains many sulfur compounds, but the ones most responsible for garlic breath are: diallyl disulfide, allyl methyl disulfide, allyl mercaptan, methyl mercaptan, and allyl methyl sulfide (AMS). The gases released by all of these compounds, except forAMS, originate in the oral cavity when we mechanically crush garlic in our mouths, so brushing your teeth and tongue will reduce the presence of the mouth-originated odors. However, good dental hygiene doesn’t usually entirely get rid of the smell because AMS is what causes unwelcome garlic breath, and this can linger for several hours or even days.

Allyl Methyl Sulfide (AMS), the unwanted pungent houseguest that overstays its welcome. — Background Credit: Crispin Semmens (conskeptical/Flickr)
AMS is a sulfur compound formed inside the body from allyl mercaptan, so instead of originating in the mouth, AMS is produced in the microflora of the gut. The resultant gas quickly evaporates into the bloodstream, which then diffuses to the lungs and infuses each breath of air that leaves our bodies with traces of strong-smelling allyl methyl sulfide. And if that isn’t wonderful enough, the compound is also released through pores of the skin, which is why you may notice a lingering body odor after garlic-heavy meals. Unfortunately, AMS does not get metabolized in your gut and liver like many other molecules that we eat, so it takes much longer for AMS to breakdown – which is why the AMS stays in the body for many hours later. [1]
SOLUTIONS: When brushing your teeth (sadly) isn’t enough
-
EAT THIS: Parsley, Spinach, Mint, Apples, Pears, plus any fruits and veggies that are prone to browning (think avocados, bananas, potatoes, etc.)
WHY: These foods contain an enzyme called polyphenol oxidase. (The same enzyme is what makes your fruit salad look brown!). When this compound is exposed to oxygen, it reacts in a way that reduces both the odors of the volatile compounds and the formation of more AMS. [2]
-
DRINK THIS: Green Tea, Coffee, Ku-Ding-Cha (a bitter-tasting Chinese tea), Prune Juice
WHY: These drinks contain a polyphenolic compound called chlorogenic acid, which is another chemical that works to deodorize garlic-derived sulfur compounds on human breath. [2]
-
ALSO DRINK THIS: Lemon juice, Soft Drinks, Beer, Hot Cocoa (and other acidic foods/beverages)
WHY: When garlic cloves are cut or crushed open, they release an enzyme called alliinase that facilitates the reactions which produce compounds responsible for the smell of garlic. Because these drinks have a pH below 3.6, they quickly destroy alliinase and minimize the formation of garlic volatiles. [2]
-
DRINK THIS INSTEAD OF WATER: Milk!
WHY: While drinking water works extremely well for reducing garlic breath, milk works even better because of its extra fat, protein, and sugar. Specifically, whole milk is effective in the reduction of the hydrophobic compounds diallyl disulfide and allyl methyl disulfide because of its high fat content. Note that drinking milk during a garlic-heavy meal does a better job of killing garlic breath than drinking milk afterwards, because the milk is able to directly react with the volatile compounds when it is mixed with garlic. [3]
Makes me think garlic ice cream might actually be a genius all-in-one odor-neutralizing dessert!
References Cited:
-
Suarez, F., Springfield, J., Furne, J., Levitt. M. Differentiation of mouth versus gut as site of origin of odoriferous breath gases after garlic ingestion. Am J Physiol. 1999; 276(2):425–30.[http://ajpgi.physiology.org/content/276/2/G425]
-
Munch, R., Barringer, S.A. Deodorization of Garlic Breath Volatiles by Food and Food Components. Journal of Food Science. March 2014; 79(4): C536-533.
-
Hansanugrum, A. Barringer, S.A. Effect of Milk on the Deodorization of Malodorous Breath after Garlic Ingestion. Journal of Food Science. August 2010; 75(6): C549-558.
 About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.
About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.
Insect Farmers & Kitchen Scientists
/in What We're Reading/by Grant AlkinLarry Peterman of Hotlix insect-containing candies gives insight into insect farming, and Guy Crosby of Cooks Illustrated helps shed a scientific light on the mysteries of the kitchen.
Read more
Milk: From Breast to Cheese with Dan Drake
/in Course Lectures/by Grant AlkinVeterinarian and goat cheese expert Dan Drake introduced UCLA students to the science of cheesemaking as part of our 2013 Science and Food course. Did you know that good cheese starts with healthy, happy goats? Check out the highlights:
 About the author: Vince C Reyes earned his Ph.D. in Civil Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.
About the author: Vince C Reyes earned his Ph.D. in Civil Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.


![Melted Cheese [Photo Credit: Pittaya Sroilong]](https://scienceandfooducla.files.wordpress.com/2015/10/2358110735_eb1d371e4e_o.jpg?w=660)