The Science of Pie – June 1, 2014
Honorable Mention Pie
Alexis Cary & Matthew Copperman (Team On the Road)
If you’ve baked an apple pie, you have probably encountered the dreaded problem of a soggy pie crust.
The student scientists of Team On the Road sought to solve this pie-baking mishap by determining the optimal apple slice thickness; the idea was that apple slices of varying thickness would release different amounts of water when baked, with more water released giving rise to a soggy crust. To investigate the effect of apple slice thickness, they cooked apples of different slice geometries, and measured the “elastic modulus”, which is how much the apple pieces deform in response to a given applied mass.

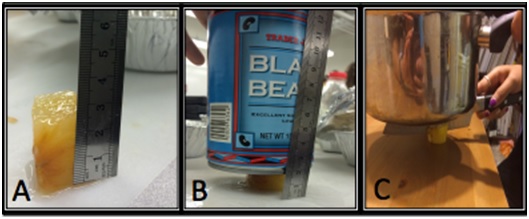
(A) Copperman rolls out the pie crust while Cary prepares ingredients. (B) The team presents their pie and poster at the 2014 Science of Pie event. (C) The team tested the elastic modulus of apple slices of varying thicknesses. Photos (A) and (B) courtesy of Patrick Tran. Photo (C) courtesy of Team On the Road.
The team prepared five different samples of apple slices with thicknesses: 3mm, 6mm, 9mm, 12mm, and 15mm. The apples of each thickness group were recorded for mass and elastic modulus before and after being baked for 20 minutes at 375°F.
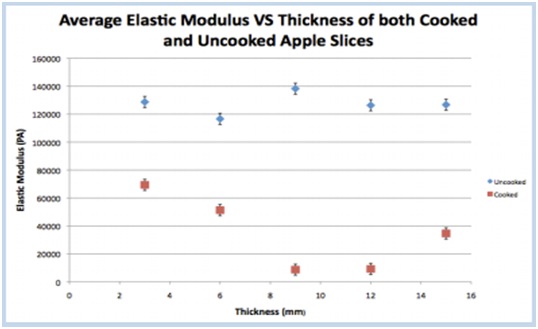
The elastic modulus is shown as a function of slice thickness for uncooked (blue) and cooked (red) apples.
The thinnest apple slices (3mm) had the least change in elastic modulus. In fact, as the slices increased in thickness, they showed increased deformation in apple shape and texture after cooking. There is an exception for the thickest apple slice (15mm), which the team attributes to the thickest apples not being fully cooked in 20 minutes. Because the thinnest apple slices maintained their firm texture and released the least amount of water, Team On the Road used extremely thin apple slices in their final pie. Soggy pie crusts, begone! Thin apple slices are here to save the day!
Note: While the team cut their slices by hand, we recommend using a mandoline to achieve uniformly thin slices of applies.
Recipe
Perfectly Unsoggy Classic Apple Pie
For the crust:
2 1/2 cups unbleached all-purpose flour, plus extra for dusting
2 tablespoons granulated sugar
1 teaspoon table salt
4 tablespoons cold vegetable shortening, cut into 8 pieces
16 tablespoons cold unsalted butter, cut into 16 pieces
6 – 8 tablespoons ice water
For the filling:
3/4 cup granulated sugar
2 tablespoons all-purpose flour
1 teaspoon lemon zest from 1 medium lemon
1/4 teaspoon table salt
1/4 teaspoon ground nutmeg
1/4 teaspoon ground cinnamon
1/8 teaspoon ground allspice
1 lemon’s worth of lemon juice
2 pounds Granny Smith apples, peeled, cored, and sliced as thin as possible (approx. 1/8” is as small as this team consistently achieved.)
1 pounds Gala apples, peeled, cored, and sliced the same as the Granny Smith Apples
For assembly:
1 egg white, beaten lightly
1 tablespoon granulated sugar, for topping
Adjust oven rack to lowest position, place rimmed baking sheet on rack, and heat oven to 400 °F.
To prepare the crust:
Process flour, sugar, and salt together in food processor until combined, about 5 seconds. Scatter shortening over top and pulse mixture for 5 times, 2 seconds each pulse.
Scatter butter over top and pulse mixture until it resembles coarse crumbs, about 10 pulses. Transfer mixture to large bowl.
Add 3 tablespoons ice water over the mixture. Stir and press dough together. (The team used a stiff rubber spatula.) Add 3 more tablespoons of water and mix until dough sticks together. Continue to add remaining ice water, less than 1 tablespoon at a time, as needed until the dough comes together.
Divide dough into two even pieces. Next, it is very helpful to lightly flour counter, hands, and rolling pin. Roll out dough into 12-inch diameter circles and transfer one of the circles into pie pan. Let excess dough hang over the edge. Press dough lightly into the bottom, corners and edges of pan.
Wrap in plastic wrap and refrigerate for at least 1 hour. Wrap the other pie crust dough in plastic wrap and refrigerate. Refrigeration is important for allowing gluten strands to relax (so the dough becomes easier to roll out), and for letting letting liquids incorporate to moisturize the dough.
To prepare filling:
Mix sugar, flour, lemon zest, salt, nutmeg, cinnamon, and allspice together in large bowl. Add lemon juice and apples and toss until combined. Let apples sit in mixture for 5-10 minutes.
To assemble the pie:
Remove pie dough from refrigerator.
Pour apples into the dough-lined pie pan, adding about half of the liquid from the apple mixture to the pie pan. Spread apples so that they create a slight mound in the middle.
Loosely roll remaining dough round around rolling pin and gently unroll it onto filling. Trim overhang to 1/2 inch beyond lip of pie plate. Pinch edges of top and bottom crusts firmly together, pressing overhanging dough towards the pie pan until it lies flush with the pan.
Crimp dough evenly around edge of pie using your fingers. Cut a 2-inch “X” into the upper crust. Brush surface with beaten egg white and sprinkle evenly with remaining 1 tablespoon sugar.
Place pie on heated baking sheet, and bake for 30 minutes. Rotate pie and bake for an additional 30 minutes. Crust should be golden brown. If necessary cook for up to 10 minutes longer.
Let pie cool on wire rack. Serve at room temperature.
 About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.
About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.
Read more by Eunice Liu




![Melted Cheese [Photo Credit: Pittaya Sroilong]](https://scienceandfooducla.files.wordpress.com/2015/10/2358110735_eb1d371e4e_o.jpg?w=660)