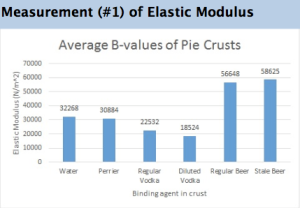
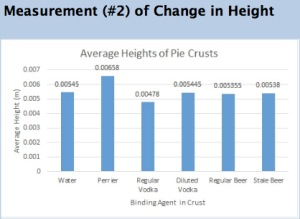
Could Making Beer From Sewage Save Us From The Drought?
To be clear, the brewers did not make beer straight from water entering out of the toilets and sewers of Oregon. Clean Water Services provided the brewers with “ultrapure water” for making their beer. Ultrapure water is made from water that is purified using the most advanced water treatment methods available. Ultrapure water is not new, but is normally not used for brewing. It is traditionally used for generating water for electronics and pharmaceuticals production, scientific research, or any other application where water must be free from as many contaminants as possible.
To generate ultrapure water, Clean Water Services combines traditional wastewater treatment with more advanced methods. For this process, sewage is first cleaned using traditional wastewater treatment, which includes screening, sedimentation, biological treatment, and disinfection. After this step, the sewage is fit to be released to lakes and rivers, but gets a deeper cleaning through more advanced methods. In the case of the water used by the brewers, Clean Water Services uses a three-step process of Ultrafiltration, Reverse Osmosis, and Enhanced Oxidation to produce their ultrapure water.
The water is first subject to Ultrafiltration and Reverse Osmosis. These processes work like a kitchen sieve as they push water through small pores in a barrier to separate water from different molecules. While both Ultrafiltration and Reverse Osmosis use similar physical separation mechanisms, they vary in the products they can remove from water because of their differing pore sizes. Ultra-filtration can be used to remove particles as small as viruses and bacteria (0.005 – 0.5 μm), while Reverse Osmosis uses finer pores, which can remove even smaller molecules like herbicides, pesticides, salts, and metal ions (0.0001 – 0.001 μm) (Figure 1).

Figure 1: The size of materials that can be removed by Ultrafiltration and Reverse osmosis. [Figure Credit: Designerwater.co]
H2O2 + UV -> 2·OH
A hydroxyl radical is just a hydrogen atom bonded to an oxygen atom with an extra electron. Having an extra electron makes hydroxyl radicals very reactive and can break down undesirable molecules in water. This final step removes any remaining contaminants that were not eliminated by Ultrafiltration and Reverse Osmosis.
![Figure 2: Ultrapure water (high purity water) compared to river water, cleaned sewage water, and tap water. [Image Credit: huffingtonpost.com]](https://scienceandfooducla.files.wordpress.com/2015/05/o-water-samples-570.jpg)
Figure 2: Ultrapure water (high purity water) compared to river water, cleaned sewage water, and tap water. [Image Credit: huffingtonpost.com]
Currently in the U.S., recycled water typically cannot be used directly as drinking water, regardless of how much it is cleaned. Generally, recycled water is only used to water landscape, cool power plants, or flush the toilet. But with growing concerns over shrinking water sources, these views are changing. In 2010, a study by the California State Water Board examined the potential contaminants in recycled water, current water treatment technology, and human health studies of exposure to these contaminants. The conclusion was that recycled water could be safe for human consumption4. These results have been confirmed by other research as well5.
Projects like this may cause you to re-evaluate your bias about the source of your water (and beer). Regardless of the origin of your water, advances in water treatment technologies may enable us to produce safe drinking water from wastewater. But the question still remains: would you feel comfortable raising a glass of beer made from recycled waste water to your lips or would you pour it down the drain?
Learn More
- “Water and Wasterwaster: Treatment/Volume Reduction Manual” Brewers Association.
- “Is Sewage Beer The Next Big Thing?” Huffington Post.
- “To Grow A Craft Beer Business, The Secret’s In The Water” NPR: The Salt.
- “Final Report: Monitoring Strategies for Chemicals of Emerging Concern (CECs) in Recycled Water” State Water Resources Control Board.
- Rodriguez, C., Buynder, P.V., Lugg, R., Blair, P., Devine, B., Cook, A., Weinstein, P. Indirect Potable Reuse: A Sustainable Water Supply Alternative. International Journal of Environmental Research and Public Health. March 2009; 6(3): 1174-1209.
 About the author: Vince C Reyes earned his Ph.D. in Civil Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.
About the author: Vince C Reyes earned his Ph.D. in Civil Engineering at UCLA. Vince loves to explore the deliciousness of all things edible.


![[Photo Credit: Vince C Reyes]](https://scienceandfooducla.files.wordpress.com/2015/05/screen-shot-2015-05-04-at-7-35-26-pm-copy.jpg)

![[Photo Credit: Vince C Reyes]](https://scienceandfooducla.files.wordpress.com/2015/01/img_0812.jpg?w=660)
![Figure 1: Molecular Formula of Ethanol [Image Credit: Vince C Reyes]](https://scienceandfooducla.files.wordpress.com/2015/01/ethanol.jpg?w=660)
![Figure 2: Layering in a Buttery Nipple. *ABV is not always an indicator of density. [Image Credit: Vince C Reyes]](https://scienceandfooducla.files.wordpress.com/2015/01/diagram.jpg?w=660)