
Photo credits: (flickr/Jessica Spengler)
The 68th United Nations General Assembly has declared 2016 the International Year of Pulses. [1] Pulses – that throbbing sensation of your carotid artery after a workout or during a first date, right? Nope. The UN suggests we celebrate the pulses that are leguminous crops harvested solely for their dry seeds. All lentils, and all varieties of dried beans, such as kidney beans, lima beans, butter beans and broad beans are pulses, as are chick peas, cowpeas, black-eyed peas and pigeon peas. Seeds that are harvested green, like green peas or green beans are classified as vegetable crops, not pulses. Legumes used primarily for oil extraction, like soybeans, are also not pulses. [2]
Why are pulses getting a year-long, world-wide campaign?
A global push for pulse production would address many problems of our global food system. The Food and Agriculture Organization of the United Nations’s campaign highlights these key benefits to pulse cultivation [1]:
- Pulses are highly nutritious – they are excellent plant source of protein, and contain the B vitamins that our bodies require to convert food to energy
- Pulses are economically accessible and contribute to food security at all levels – from farmers to consumers
- Pulses foster sustainable agriculture, thus addressing agriculture’s role in climate change
- Pulses promote biodiversity in agriculture
Now that we know the basics of pulses and why they’re important, let’s get scientific.

Photo credits: (flickr/Kelly Garbato)
Pulses in the nitrogen cycle
Pulses are legumes, or plants in the family Leguminosae. Thanks to their symbiosis with many members of the diazotrophic, or nitrogen-fixing bacterial genus Rhizobium that live in their roots and feed them with nitrogen from the air, pulses have a particularly high protein content compared to non-legumes. [3] Within the bacterium, atmospheric nitrogen (N2), which is typically unusable to plants, is converted to ammonium (NH4+) via the activity of the enzyme nitrogenase. The nitrogen of ammonium is converted to other more complex compounds that are beneficial to humans, like amino acids – the building blocks of protein. In exchange for fixing nitrogen, the bacterium receives food from the plant — carbon in the form of glucose (C6H12O6).
This remarkable bacterial symbiosis also enriches the soil in which pulses grow with nitrogen compounds like nitrite (NO2–) and nitrate (NO3–), which is the preferred nitrogen source for other green plants. For this reason, farmers who crop-rotate with legumes don’t need to apply nearly as much fertilizer as farmers who don’t. [3]
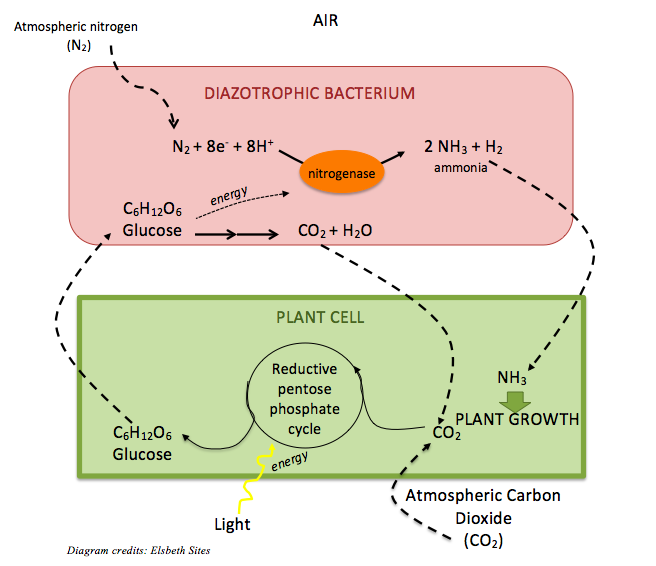
Pulses in a changing climate
Many pulses are also hardy and drought tolerant crops – lentils, broad beans, peas, and chick peas are all native to the Fertile Crescent of the Near East, and have adapted to sprout quickly and reproduce in the rainy season before the hot, dry summer [3].
Anatomy of the pulse
All food seeds consist of three basic parts: an outer protective coat, the small embryonic portion that develops into the mature plant, and the storage tissue that feeds the plant embryo. [3]The bulk of the seed consists of storage cells are filled with particles of concentrated protein and granules of starch, or organized masses of starch chains.
Cooking and starch retrogradation
When we cook pulses, hot water permeates the starch granules. As the water molecules work themselves between the starch chains, the granules swell and soften. When the pulses later cool down, the starch chains bond to each other again in tighter, more organized associations, resulting in firmer granules. (This process is called retrogradation.) [3] Consider leftover lentils or beans: they’re always harder and drier the next day, and they never get quite as soft as when they were first cooked. This is because during the process of retrogradation, some starch molecules form granules that are even more tightly associated than the bonds in the original starch granule. They form small crystalline regions that resist breaking even at boiling temperatures. [3]
Retrogradation of starch might foil your plans for leftover lentils, but it does do our bodies good: Our digestive enzymes cannot easily digest retrograded starch, so eating it results in a more gradual rise in blood sugar compared to the effects of non-retrograded starch. [3] Our intestines need help breaking down this tough starch, and the beneficial bacteria in our large intestines are happy to be of assistance. Just as the diazotrophic bacteria in soil work in harmony with leguminous plants, our intestinal bacteria digests what we cannot. Thus the retrograded starch functions as a prebiotics, or food for the probiotic bacteria in our guts. Well-fed gut bacteria make for healthy digestive tracks and happy bowels.
Will this pulse promotion save the world and fix the global food economy? Perhaps. We can all do our part by making a hearty spinach dal for dinner tonight, and sweet red bean paste for dessert.
Works Cited
- “”Save and Grow in Practice” Highlights Importance of Pulses in Crop Rotations and Intercropping.” Pulses – 2016 | 2016 International Year of Pulses. Food and Agriculture Organization of the United Nations, n.d. Web. 05 Feb. 2016.
- “What Are Pulses? | FAO.” What Are Pulses? | FAO. Food and Agriculture Organization of the United Nations, 15 Oct. 2015. Web. 05 Feb. 2016.
- McGee, Harold. “Seeds.” On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004. N. pag. Print.
 About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.
About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.
Read more by Elsbeth Sites


 About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.
About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.






