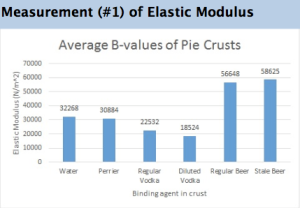
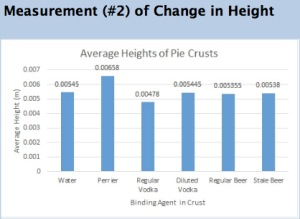
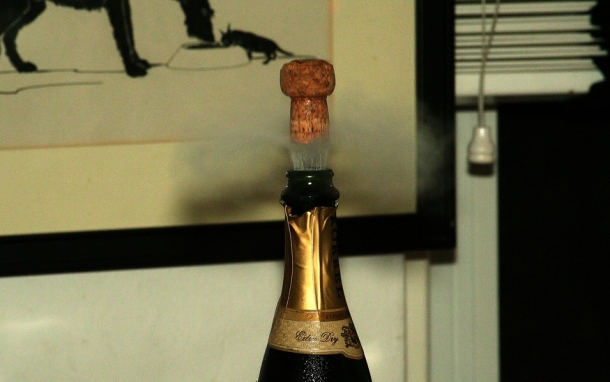Pop Rocks and Carbonation
Some might say one of life’s little pleasures is eating candy. Those who have tried Pop Rocks, however, know that its sugary glory and dare-devilish allure warrant an entirely new adventure. Although it appears harmless, a handful of Pop Rocks candy will set off a fizzy explosion of sugar crystals and popping noises in your mouth. But no remorse is needed; Pop Rocks aren’t actually dangerous. (Mythbusters proves your stomach won’t explode.)
How are Pop Rocks made?
Pop Rocks were developed by scientist William A. Mitchell in 1956 with a technique patented in 1961 to create a revolutionary confection which “enclos[es] a gas within a solid matrix” [1, 2]. Essentially, Pop Rocks is made of a typical hard candy sugar solution (sucrose, lactose, corn syrup and flavoring) with the addition of one important ingredient: highly-pressurized carbon dioxide (CO2).
First, the sugar solution is heated and melted to obtain a “fusible sugar”. Pop Rocks, like most other hard candies, uses a sugar solution of sucrose, lactose, and corn syrup, because these ingredients produce candy with low hygroscopicity – which means the candy is less likely to absorb water from the surrounding atmosphere [2]. This ensures that the sweet morsels do not dissolve as easily in a humid environment; they are also less sticky and have a longer shelf life.
Just as CO2 transforms syrupy juice into soda, it will turn ordinary candy into Pop Rocks! The way this works: CO2 at 600 pounds per square inch (psi) is mixed with the melted sugar until there is about 0.5 to 15 ml of gas per gram of sugar [1, 2]. Note that 600 psi is roughly 7 times greater than the pressure inside a champagne bottle, 20 times greater the pressure in your car tires, and 40 times greater than normal atmospheric pressure at sea level [5, 6].
Once the CO2 is thoroughly incorporated, a process which takes anywhere from 2-6 minutes [2], the mixture is cooled and the candy hardens. Cooling is done as rapidly as possible to prevent CO2 from diffusing out of the candy, reduce hygroscopicity, and minimize crystallization, a process which makes the candy very fragile. [2] This causes the Pop Rocks to shatter and gives the candy’s signature appearance, “mini rocks” of sugar crystals.
The result? Small candy pieces encapsulating bubbles of high-pressure CO2. Lo, the magic of carbonation!
So why do Pop Rocks pop?
When you eat Pop Rocks, the moisture and temperature in your mouth melts the candy. The subsequent popping sounds are a result of the high-pressure CO2 bubbles being released into atmospheric pressure! But what about the crackling sensations felt in your mouth? Why do we perceive carbonation as a fizzy, tingling flavor sensation?
In the past few years, scientists have identified that taste receptor cells can actually detect and respond to carbonation. Specifically, sour-sensing taste receptor cells are activated in response to CO2 and are responsible for the “taste of carbonation” [3].
The Taste of Carbonation
Sour-sensing taste receptors specifically express a gene which encodes carbonic anhydrase 4, which is an enzyme that catalyzes the conversion of CO2 to bicarbonate ions (HCO3–) and free protons (H+). This enzyme is only attached on the surface of sour-sensing taste receptor cells, so when you eat Pop Rocks or drink carbonated soda, CO2 is broken down and H+ proton byproducts linger outside of the cell. Since sour-sensing taste receptors activate in response to acidic environments. Therefore, they will detect this abundance of free H+ protons and ultimately, detect the taste of carbonation [3].

(A) CO2 is broken down into HCO3– and H+ by the carbonic anhydrase 4 enzyme
(B) The abundance of H+ byproducts creates an acidic environment. Through ion channels, the H+ ions enter the sour-taste receptor, which depolarizes the cell and leads to the detection of CO2 .
However, carbonation doesn’t always taste sour to us because CO2 is detected by multiple somatosensory systems in the body. Some researchers even suggest that the tingling, burning sensations associated with the perception of carbonation can be caused by CO2 triggering pain receptors [4].
Would this mean our society’s desire for carbonated food and drink has strangely evolved against a natural aversion to experiencing pain? Personally, I can’t hear over the loud buzzing noises of Pop Rocks in my mouth to find out…and as they say, “no pain, no gain”!
Note: Modified on September 19, 2014
The diagram illustrating taste detection of carbonation has been added in the current post.
References cited
- “Why do Pop Rocks pop?” http://www.poprockscandy.com/history.html. Accessed 23 August 2014.
- Leon K, Mitchell W (1961) Gasified confection and method of making the same. US Patent No. US3012893 A. Available: http://www.google.com/patents/US3012893. Accessed 20 August 2014.
- Chandrashekar J, Yarmolinsky D, von Buchholtz L, Oka Y, Sly W, et al. (2009) The Taste of Carbonation. Science 326: (5951) 443-445. doi:10.1126/science.1174601.
Available: http://www.sciencemag.org/content/326/5951/443.full. Accessed 25 August 2014. - Marziali C (2010) “Sparkling Drinks Spark Pain Circuits”. University of Southern California. http://dornsife.usc.edu/news/stories/796/sparkling-drinks-spark-pain-circuits/. Accessed 26 August 2014.
- “Champagne FAQ’s” http://www.champagnesabering.com/home.php?id=16. Accessed 16 September 2014.
- “How To Check Tire Pressure” http://www.dmv.org/how-to-guides/check-tire-pressure.php. Accessed 16 September 2014.
 About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.
About the author: Eunice Liu is studying Neuroscience and Linguistics at UCLA. She attributes her love of food science to an obsession with watching bread rise in the oven.