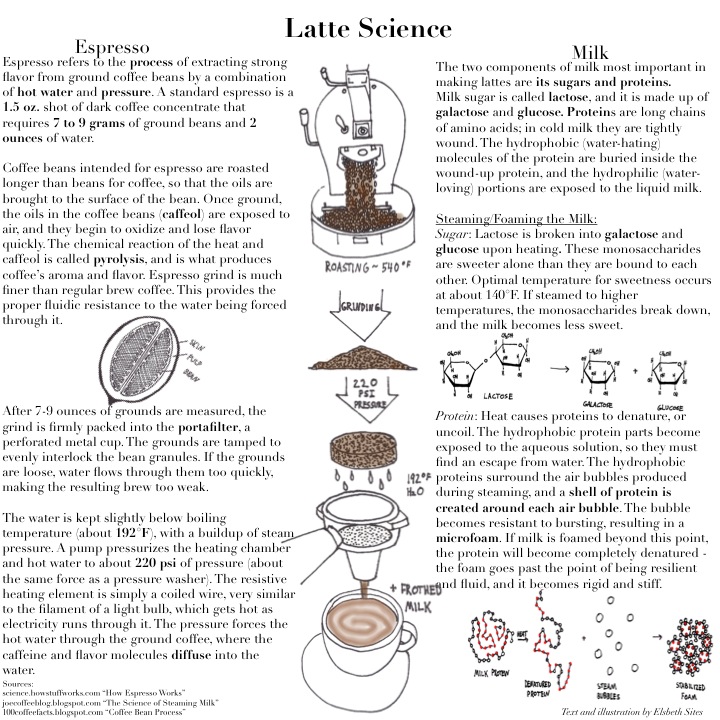
Tag Archive for: beans
Aquafaba & Other Hopes for Delicious Egg-free Meringues
/in Science & Food/by Grant AlkinMeringues are one of the few desserts that are simple yet elegant works of art. They are also precursors to other impressive, albeit considerably more complicated, desserts such as baked Alaska, lemon meringue pies, and macarons. At the bare minimum, all you need to make a fluffy meringue is egg whites, sugar, and an electric mixer—or an egg beater and some arm power. For vegans, this egg-containing dessert is not an option—but why should vegans (and those with egg allergies) miss out on this sweet, airy dollop of heaven?
To make a decent egg-free meringue, it helps to understand the meringue at the molecular level. How does a liquid get whipped into a cloud-like solid?
Egg whites, comprising 90% water, are undeniably runny. The other 10% consists of proteins, which play a major role in the fluid-to-fluff transformation. Mechanical stress from rigorously beating the egg whites causes the egg white proteins to denature, unfold from their natural structure. This exposes various amino acids, the building blocks of proteins, to the rapidly aerating environment. Some of the amino acids are hydrophobic (water-fearing), and some are hydrophilic (water-loving). As the egg whites are whipped, hydrogen bonds form between the hydrophilic amino acids and water in the egg whites. The hydrophobic amino acids prefer to be exposed to the air that is quickly beaten into the liquid mixture. Air ends up trapped in the meshwork of denatured proteins within the developing foam, and so the longer the mixture is beaten, the fluffier it gets. To retain the trapped air bubbles and generate peaks that stand up straight, sugar is added as a stabilizer. And eccola! Una nuvola dolce nella ciotola; a fluffy meringue is ready to bake or prepare into macarons or boccone dolce.
To create an equally amazing and delicious vegan counterpart, the egg whites would have to be substituted with an ingredient that has both water-loving and water-fearing parts. Logic may think to search for a plant-based protein alternative, but French chef Joël Roessel discovered that chickpea brine works perfectly well as a vegan egg-white substitute [1]. Coined aquafaba by Goose Wohlt (Latin for “bean water”), the leftover water from a can of chickpeas can be combined with sugar and whisked into a vegan meringue that surprisingly tastes nothing like beans. Of all the possible substitutions, why does aquafaba work in lieu of egg whites?

Water leftover from cooking chickpeas, also known as aquafaba, can be used in lieu of egg whites. Photo credit: getselfsufficient/Flickr
Anne Rieder, a scientist at the Norwegian food research institute Nofima, analyzed aquafaba and revealed that the bean water contains equal amounts of proteins and carbohydrates [2]. The function of proteins in the aquafaba are similar for meringue-making; Rieber suggests that the carbohydrates may serve as an additional stabilizer by increasing the viscosity of the water portion of the foam.
To create foams like meringues, Kent Kirshenbaum, a professor at NYU, was inspired by chemistry to invent a foaming agent that is rich in saponins, currently awaiting patent approval. Saponins are a class of chemicals found in plants, including beans like chickpeas. The name derives from the soapwort plant, Saponaria, which contains the Latin root for soap, sapo; this is a fitting name, given the compound’s propensity to foam when shaken in water [3]. Like the amino acids of proteins, saponin molecules contain a hydrophobic and a hydrophilic moiety that enables them to interact with both air and water.
Whatever the reason for avoiding eggs, at least you won’t have to forfeit the heavenly delight that is a lightweight meringue cookie.
References cited
- “Aquafaba history.” The Official Aquafaba Website.
- “Aquafaba, what is its chemical composition?” Frie kaker.
- “Saponins.” Cornell University Department of Animal Sciences.
 About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.
About the author: Alice Phung once had her sights set on an English degree, but eventually switched over to chemistry and hasn’t looked back since.
The International Year of Pulses
/in Science & Food/by Grant Alkin
Photo credits: (flickr/Jessica Spengler)
The 68th United Nations General Assembly has declared 2016 the International Year of Pulses. [1] Pulses – that throbbing sensation of your carotid artery after a workout or during a first date, right? Nope. The UN suggests we celebrate the pulses that are leguminous crops harvested solely for their dry seeds. All lentils, and all varieties of dried beans, such as kidney beans, lima beans, butter beans and broad beans are pulses, as are chick peas, cowpeas, black-eyed peas and pigeon peas. Seeds that are harvested green, like green peas or green beans are classified as vegetable crops, not pulses. Legumes used primarily for oil extraction, like soybeans, are also not pulses. [2]
Why are pulses getting a year-long, world-wide campaign?
A global push for pulse production would address many problems of our global food system. The Food and Agriculture Organization of the United Nations’s campaign highlights these key benefits to pulse cultivation [1]:
- Pulses are highly nutritious – they are excellent plant source of protein, and contain the B vitamins that our bodies require to convert food to energy
- Pulses are economically accessible and contribute to food security at all levels – from farmers to consumers
- Pulses foster sustainable agriculture, thus addressing agriculture’s role in climate change
- Pulses promote biodiversity in agriculture
Now that we know the basics of pulses and why they’re important, let’s get scientific.

Photo credits: (flickr/Kelly Garbato)
Pulses in the nitrogen cycle
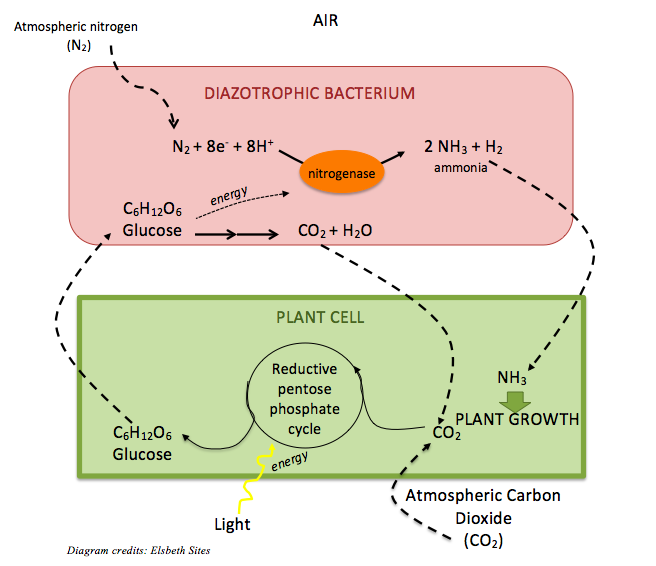
Pulses are legumes, or plants in the family Leguminosae. Thanks to their symbiosis with many members of the diazotrophic, or nitrogen-fixing bacterial genus Rhizobium that live in their roots and feed them with nitrogen from the air, pulses have a particularly high protein content compared to non-legumes. [3] Within the bacterium, atmospheric nitrogen (N2), which is typically unusable to plants, is converted to ammonium (NH4+) via the activity of the enzyme nitrogenase. The nitrogen of ammonium is converted to other more complex compounds that are beneficial to humans, like amino acids – the building blocks of protein. In exchange for fixing nitrogen, the bacterium receives food from the plant — carbon in the form of glucose (C6H12O6).
This remarkable bacterial symbiosis also enriches the soil in which pulses grow with nitrogen compounds like nitrite (NO2–) and nitrate (NO3–), which is the preferred nitrogen source for other green plants. For this reason, farmers who crop-rotate with legumes don’t need to apply nearly as much fertilizer as farmers who don’t. [3]
Pulses in a changing climate
Many pulses are also hardy and drought tolerant crops – lentils, broad beans, peas, and chick peas are all native to the Fertile Crescent of the Near East, and have adapted to sprout quickly and reproduce in the rainy season before the hot, dry summer [3].
Anatomy of the pulse
All food seeds consist of three basic parts: an outer protective coat, the small embryonic portion that develops into the mature plant, and the storage tissue that feeds the plant embryo. [3]The bulk of the seed consists of storage cells are filled with particles of concentrated protein and granules of starch, or organized masses of starch chains.
Cooking and starch retrogradation
When we cook pulses, hot water permeates the starch granules. As the water molecules work themselves between the starch chains, the granules swell and soften. When the pulses later cool down, the starch chains bond to each other again in tighter, more organized associations, resulting in firmer granules. (This process is called retrogradation.) [3] Consider leftover lentils or beans: they’re always harder and drier the next day, and they never get quite as soft as when they were first cooked. This is because during the process of retrogradation, some starch molecules form granules that are even more tightly associated than the bonds in the original starch granule. They form small crystalline regions that resist breaking even at boiling temperatures. [3]
Retrogradation of starch might foil your plans for leftover lentils, but it does do our bodies good: Our digestive enzymes cannot easily digest retrograded starch, so eating it results in a more gradual rise in blood sugar compared to the effects of non-retrograded starch. [3] Our intestines need help breaking down this tough starch, and the beneficial bacteria in our large intestines are happy to be of assistance. Just as the diazotrophic bacteria in soil work in harmony with leguminous plants, our intestinal bacteria digests what we cannot. Thus the retrograded starch functions as a prebiotics, or food for the probiotic bacteria in our guts. Well-fed gut bacteria make for healthy digestive tracks and happy bowels.
Will this pulse promotion save the world and fix the global food economy? Perhaps. We can all do our part by making a hearty spinach dal for dinner tonight, and sweet red bean paste for dessert.
Works Cited
- “”Save and Grow in Practice” Highlights Importance of Pulses in Crop Rotations and Intercropping.” Pulses – 2016 | 2016 International Year of Pulses. Food and Agriculture Organization of the United Nations, n.d. Web. 05 Feb. 2016.
- “What Are Pulses? | FAO.” What Are Pulses? | FAO. Food and Agriculture Organization of the United Nations, 15 Oct. 2015. Web. 05 Feb. 2016.
- McGee, Harold. “Seeds.” On Food and Cooking: The Science and Lore of the Kitchen. New York: Scribner, 2004. N. pag. Print.
 About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.
About the author: Elsbeth Sites received her B.S. in Biology at UCLA. Her addiction to the Food Network has developed into a love of learning about the science behind food.