Ceviche
Through the process of cooking, molecular transformations alter the macroscopic properties of our food. Consider what happens when you fry an egg: the transparent, liquid egg whites become an opaque white solid. These striking changes in the egg’s color and texture are a result of protein denaturation.
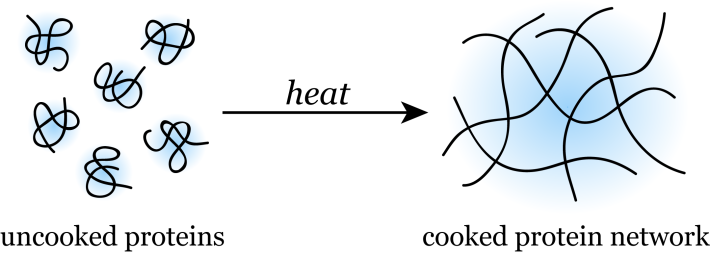
A raw egg white is essentially a suspension of proteins in water. These proteins are made of long chains of amino acids that “fold” into specific and stable three-dimensional structures. Heating the proteins in an egg white causes them to “unfold” and no longer maintain their inherent structure. In this state, proteins are said to be denatured and will readily stick to one another to form an extensive protein network.
The formation of such denatured protein networks underlies many of the macroscopic changes we observe during the cooking of protein-rich foods such as eggs and meat. The extent of protein network formation affects the final texture of cooked meat and eggs. A steak cooked medium-well, for example, will have a denser protein network that gives rise to a tougher steak than one that is cooked medium-rare [1]. Denatured protein networks also affect the optical properties of protein-rich foods. Because a cooked protein network scatters light more effectively than a suspension of uncooked proteins, eggs and fish become more opaque as they are cooked [2].
We usually associate cooking with heat-related processes, but there are other ways to denature proteins. For example, you can “cook” an egg in acetic acid:
http://www.youtube.com/watch?v=KJSwlVOt12w
This form of chemical cooking relies on high salt concentrations or extreme pH conditions to denature proteins and “cook” food. Although an egg “cooked” in pure acetic acid may not have broad taste appeal, chemical cooking is used to prepare a variety of edible dishes including brined salmon (lox), pickled herring, and lutefisk.
In this recipe for ceviche, we will use an acidic (low pH) marinade to “cook” fish without heat.
Ingredients
1 pound of previously frozen* fish, 1 inch dice.
1 cup lime juice
1 cup diced tomatoes
1/2 cup diced red onion
1/2 cup roughly chopped cilantro
Kosher salt, to taste
1 avocado, sliced
*As a safety precaution, only use fish that has been previously frozen, as freezing reduces the risk of exposure to parasites in seafood [3].
Procedure
1. In a non-reactive dish, such as a glass bowl, toss together the fish, lime juice, and garlic.
2. Marinate for 30 minutes on ice or in the refrigerator.
Lime juice contains high concentrations of natural acids like citric acid and ascorbic acid. The pH of lime juice is around 2.5—more acidic than vinegar and similar to the pH of stomach acid. As the lime juice diffuses into the fish, its low pH will cause the proteins in the fish to denature and form protein networks. As a result, the fish will become tougher and more opaque.
Believe it or not, you can actually overcook your fish with lime juice! Leaving it too long will not only make it tough and dry, but it will also break down the connective tissue, causing your fish to fall apart (more on this from The Food Lab).
3. Once marinated, add the tomatoes, onion, cilantro, and salt to the fish.
Tomatoes provide extra acidity, but by now your fish should already be “cooked.”
4. Garnish with slices of avocado and eat with tortilla chips.
Online Resources
- Recipe adapted from Keith Famie’s Ceviche
- The Food Lab: Ceviche and the Science of Marinades
- Heat changes protein structure: frying an egg animation
References Cited
- Bouton PE, Harris PV (1972) THE EFFECTS OF COOKING TEMPERATURE AND TIME ON SOME MECHANICAL PROPERTIES OF MEAT. Journal of Food Science 37: 140–144. doi:10.1111/j.1365-2621.1972.tb03404.x
- Anfinsen CB (1995) Advances in protein chemistry. Volume 47. San Diego; London: Academic Press. p. Available:http://site.ebrary.com/id/10240101. Accessed 20 January 2013.
- US Food and Drug Administration (2012) Food Facts: Fresh and Frozen Seafood: Selecting and Serving it Safely. Available:http://www.fda.gov/food/resourcesforyou/consumers/ucm077331.htm. Accessed 21 January 2013.
 About the author: Liz Roth-Johnson is a Ph.D. candidate in Molecular Biology at UCLA. If she’s not in the lab, you can usually find her experimenting in the kitchen.
About the author: Liz Roth-Johnson is a Ph.D. candidate in Molecular Biology at UCLA. If she’s not in the lab, you can usually find her experimenting in the kitchen.